BCR-ABL p190/p210 kit
Product Highlights
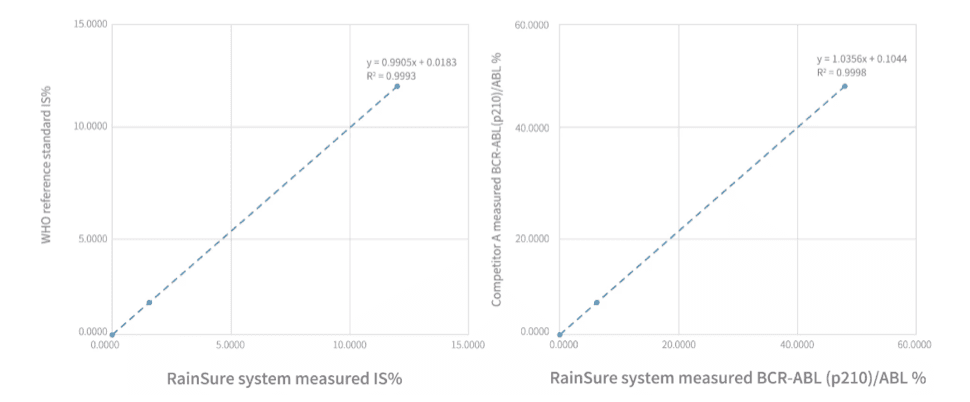
Absolute Quantification
No standard calibration curve required.Direct quantification of Molecular
High Sensitivity
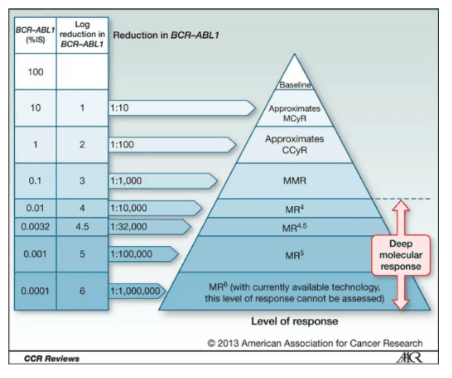
Measure MR value down to the MR 5.0 level
Simple Workflow
Automatic digital PCR system without complex manual operation
Standardized Interpreted Output
Direct reporting on the International Scale(%IS)and Molecular Response(MR) Values
Product Description
The Human BCR-ABL (P210) Fusion Gene Detection Kit (Digital PCR) is used to quantify the proportion of the BCR-ABL (P210) fusion gene in human peripheral blood specimens obtained from patients previously diagnosed with t(9:22)-positive chronic myeloid leukemia (CML).
The kit measures the e13a2 and/or e14a2 transcripts of the BCR-ABL fusion gene and normalizes them to the ABL1 endogenous control. The results are reported as a percentage reduction from a baseline of 100% on the International Scale (%IS) and on a logarithmic molecular reduction (MR) scale.
Detecting BCR-ABL (P210) (e13a2 and/or e14a2) transcript levels during tyrosine kinase inhibitor (TKI) therapy in patients with chronic myeloid leukemia (CML) is crucial for evaluating treatment response and monitoring early relapse, which is essential for optimizing CML treatment.
Please note that the test does not distinguish between e13a2 and/or e14a2 fusion transcripts and does not monitor other rare fusion transcripts resulting from t(9;22).
Why BCR-ABL assay?
About Chronic Myelogenous Leukemia (CML)
Chronic myelogenous leukemia (CML) is a malignant disease of hematopoietic stem cells characterized by myeloid proliferation. It is also called chronic myeloid leukemia (CML). The annual incidence of CML worldwide is (1–2)/100,000, accounting for 15%–20% of all adult leukemias.
Chronic myeloid leukemia (CML) has a mature program from disease diagnosis to treatment and efficacy monitoring. TKI treatment has increased the 10-year survival rate of CML patients to 85% to 90%. Patients not only pursue major molecular response (MMR), but also emphasize early molecular response (EMR) and stable deep molecular response (DMR).
Clinical practice shows that molecular response evaluation of CML patients can be achieved by monitoring minimal residual disease (MRD) at the molecular level. Therefore, standardized molecular MRD monitoring of CML patients is crucial to evaluate whether the best treatment effect is achieved. MRD detection at the appropriate time point has become a routine method in the treatment of CML.
Product Specifications
Performance information
Sample Number | RainSure dPCR BCR- ABL Copy Number (copies/uL) | RainSure dPCR ABL Copy Number (copies/uL) | RainSure dPCR Mutation Ratio (%) | RT-PCR BCR-ABL Copy Number | RT-PCR ABL Copy Number | RT-PCR Mutation Ratio |
M20-29208 | 10.16 | 170720 | 0.00595 | 0 | 302000 | 0 |
M20-28822 | 1.84 | 397600 | 0.00046 | 0 | 526000 | 0 |
M2020106 | 35360.00 | 505120 | 7.00032 | 212000 | 923000 | 22.965 |
M202011246 | 493520.00 | 714080 | 69.11270 | 1360000 | 757000 | 179.000 |
M2021-00470 | 1.84 | 388720 | 0.00047 | 0 | 250000 | 0 |